Intro To Intermolecular Forces Pogil Answers / Intermolecular forces are not as strong as intramolecular forces, but they influence a lot of properties in a chemical.. Greater the intermolecular forces, higher is the boiling point. Rank the following from weakest intermolecular forces to strongest. Do not use commas or scientific notation when entering large numbers. 1 name date block pogil: This one was quite a bit. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. London dispersion forces are attractive forces that exist between all atoms and molecules. It attracts the molecules to stick together or repulses the. The intermolecular forces arises due to following interactions: Worksheet 12a (intro) intermolecular forces predict the type of solids (ionic, molecular, or atomic) the following substances would form: Molecules do not exist as independent units: Greater the intermolecular forces, higher is the boiling point. Intermolecular forces allow us to determine which substances are likely to dissolve in which other substances and what the melting and boiling points of substances are. What is an intermolecular force? Intermolecular forces (imf) (or secondary forces) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between atoms and other types of. London dispersion forces are attractive forces that exist between all atoms and molecules. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. What are these intermolecular forces? Transcribed image text from this question. 1 name date block pogil: 1.what specific molecule is represented inside. Worksheet 12a (intro) intermolecular forces predict the type of solids (ionic, molecular, or atomic) the following substances would form: Intermolecular forces are the forces. They are a weak electrostatic force and they are caused by the movement of charge. Rank the following from weakest intermolecular forces to strongest. How much heat is required to melt 42.7 g of diethyl ether? Different types of intermolecular forces (forces between molecules). The strongest intermolecular force is hydrogen bonding which is the force of attractiong between a h atom which is covalently bonded to the lone pair of one of the strongest intermolecular forces (i.e. As you have learned, matter is made up of discrete particles called atoms, which chemically combine to form molecules. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Thank you for submitting your answer. Ø only polar species are involved in intermolecular attractive forces should be drawn between two ends of opposite charge. Van der waal forces 2. Intermolecular forces, which are weaker but hold separate molecules together. Molecules do not exist as independent units: This one was quite a bit. Do not use commas or scientific notation when entering large numbers. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Do not type units into the answer boxes, type only the numeric values. Intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules. Which of these is not an intermolecular force? Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. What are these intermolecular forces? Taken from the answer key learn with flashcards, games and more — for free. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. These can generally be overcome by physical changes such as temperature. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. In fact, groups of molecules stick. Which of the following is not a kind of intermolecular force? The strongest intermolecular force is hydrogen bonding which is the force of attractiong between a h atom which is covalently bonded to the lone pair of one of the strongest intermolecular forces (i.e. The more intermolecular forces, the higher the boiling point. There are three types of intermolecular forces; Transcribed image text from this question. Do not use commas or scientific notation when entering large numbers. What is an intermolecular force? They are also responsible for the formation of the condensed phases, solids and liquids. Forces which exist within same molecule or a polyatomic ion ,affect the chemical properties of the substance. Intermolecular forces are the forces.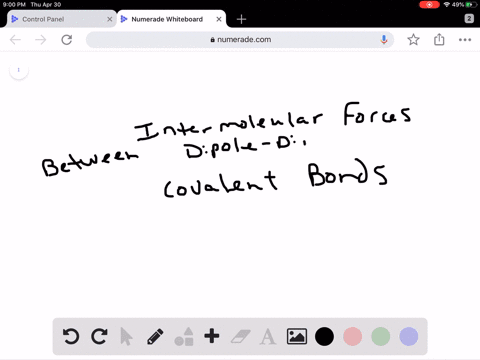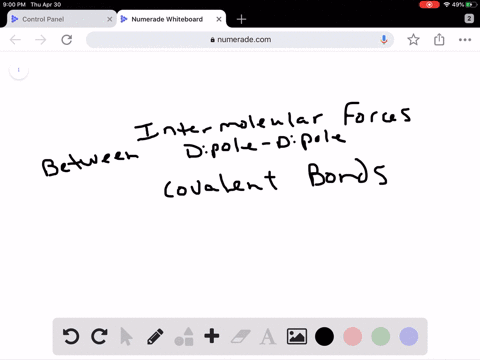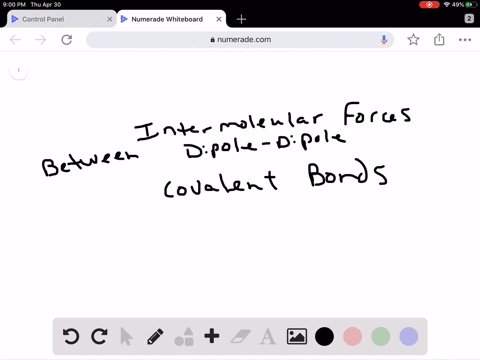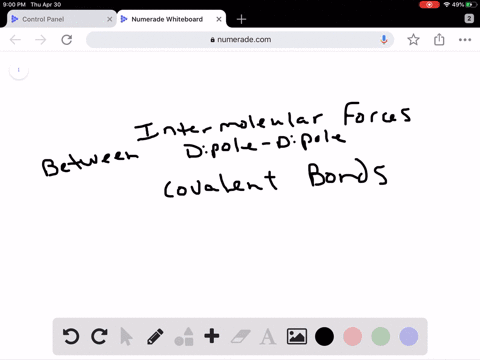
Different types of intermolecular forces (forces between molecules).
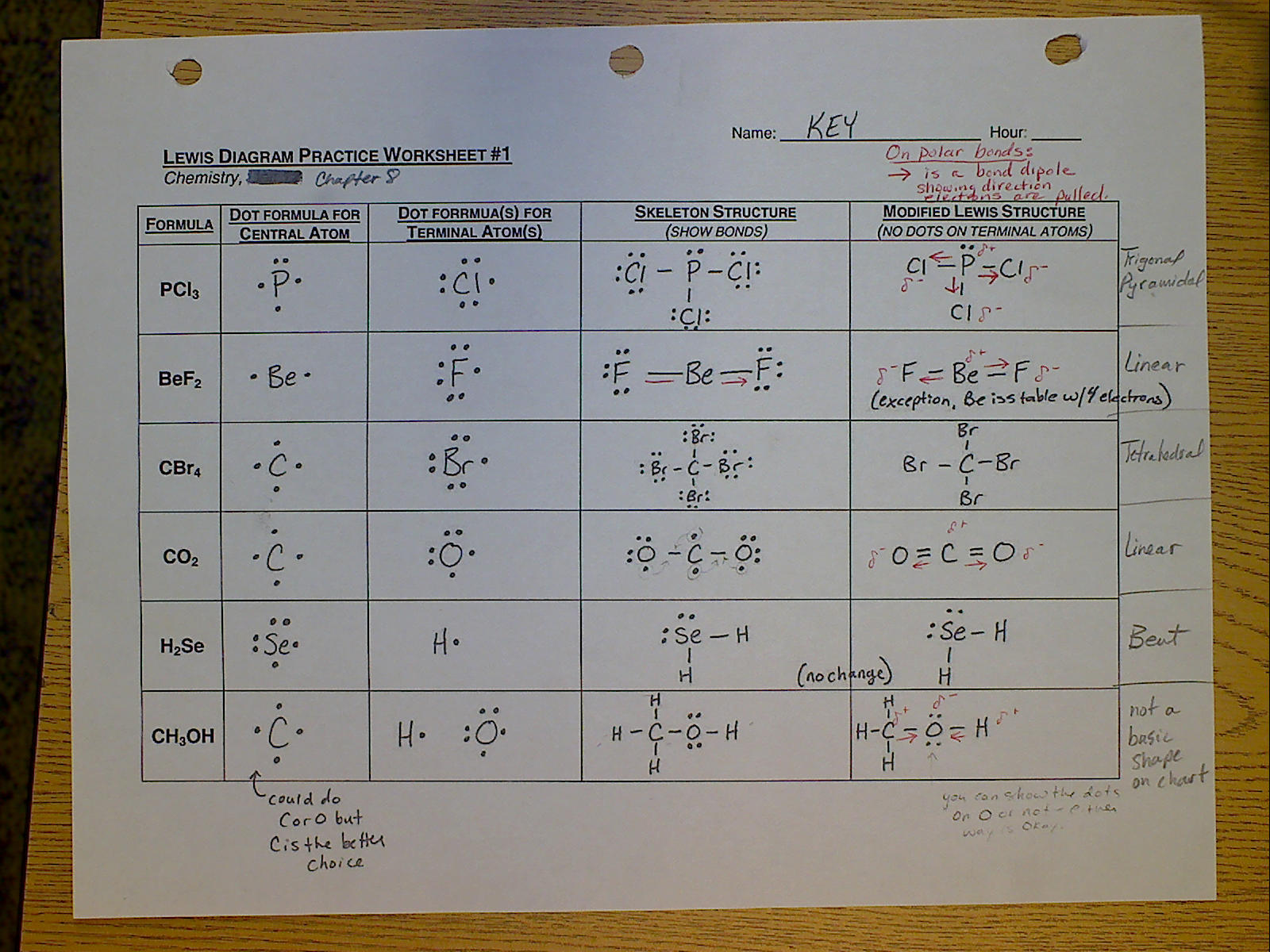
They are also responsible for the formation of the condensed phases, solids and liquids.

London dispersion forces are attractive forces that exist between all atoms and molecules.
Intro To Intermolecular Forces Pogil Answers / Intermolecular forces are not as strong as intramolecular forces, but they influence a lot of properties in a chemical.. Greater the intermolecular forces, higher is the boiling point. Rank the following from weakest intermolecular forces to strongest. Do not use commas or scientific notation when entering large numbers. 1 name date block pogil: This one was quite a bit.
Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. London dispersion forces are attractive forces that exist between all atoms and molecules. It attracts the molecules to stick together or repulses the. The intermolecular forces arises due to following interactions: Worksheet 12a (intro) intermolecular forces predict the type of solids (ionic, molecular, or atomic) the following substances would form:

Different types of intermolecular forces (forces between molecules).
Molecules do not exist as independent units: Greater the intermolecular forces, higher is the boiling point. Intermolecular forces allow us to determine which substances are likely to dissolve in which other substances and what the melting and boiling points of substances are. What is an intermolecular force? Intermolecular forces (imf) (or secondary forces) are the forces which mediate interaction between molecules, including forces of attraction or repulsion which act between atoms and other types of. London dispersion forces are attractive forces that exist between all atoms and molecules. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. What are these intermolecular forces? Transcribed image text from this question. 1 name date block pogil: 1.what specific molecule is represented inside. Worksheet 12a (intro) intermolecular forces predict the type of solids (ionic, molecular, or atomic) the following substances would form: Intermolecular forces are the forces.
They are a weak electrostatic force and they are caused by the movement of charge. Rank the following from weakest intermolecular forces to strongest. How much heat is required to melt 42.7 g of diethyl ether? Different types of intermolecular forces (forces between molecules). The strongest intermolecular force is hydrogen bonding which is the force of attractiong between a h atom which is covalently bonded to the lone pair of one of the strongest intermolecular forces (i.e.

They are also responsible for the formation of the condensed phases, solids and liquids.
As you have learned, matter is made up of discrete particles called atoms, which chemically combine to form molecules. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Thank you for submitting your answer. Ø only polar species are involved in intermolecular attractive forces should be drawn between two ends of opposite charge. Van der waal forces 2. Intermolecular forces, which are weaker but hold separate molecules together. Molecules do not exist as independent units: This one was quite a bit. Do not use commas or scientific notation when entering large numbers. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. Do not type units into the answer boxes, type only the numeric values. Intermolecular forces are forces that act between stable molecules or between functional groups of macromolecules. Which of these is not an intermolecular force?
Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. What are these intermolecular forces? Taken from the answer key learn with flashcards, games and more — for free. Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. These can generally be overcome by physical changes such as temperature.

London dispersion forces are attractive forces that exist between all atoms and molecules.
Dipoles are stronger than london forces alone, so polar molecules tend to have stronger intermolecular forces than nonpolar molecules of a similar size and polarity. Intermolecular forces determine bulk properties such as the melting points of solids and the boiling points of liquids. In fact, groups of molecules stick. Which of the following is not a kind of intermolecular force? The strongest intermolecular force is hydrogen bonding which is the force of attractiong between a h atom which is covalently bonded to the lone pair of one of the strongest intermolecular forces (i.e. The more intermolecular forces, the higher the boiling point. There are three types of intermolecular forces; Transcribed image text from this question. Do not use commas or scientific notation when entering large numbers. What is an intermolecular force? They are also responsible for the formation of the condensed phases, solids and liquids. Forces which exist within same molecule or a polyatomic ion ,affect the chemical properties of the substance. Intermolecular forces are the forces.
0 comments:
Post a Comment